Weathering
Salt Weathering

Physico-Chemical Salt Weathering
-
•Hydration of clay minerals with saline moisture causing volume change. Salt crystallization pressures and the swelling of minerals by hydration can cause cavity roofs and backwalls to flake off. The swelling and contraction of clay minerals may enhance water retention and promote flaking of clay minerals. Pye and Mottershead (1995) found that chlorine derived from sea spray is present in clays on weathered tafoni walls. When clays absorb chloride derived from saline rich fog, the properties of the clay are altered in a way that promotes water retention and flaking.
Similarly, Bradley, Hutton, & Twidale (1978) found that salts such as halite and gypsum cause small-scale flaking of the inner tafoni walls on granitic rocks in Australia. The salts released from the host rock minerals during chemical weathering are primarily Na+, K+, Ca++, and Mg++ cations. They argued for an inherent relationship between salt and flaking (and therefore differential weathering and tafoni development) because they found salt concentration in flakes 2-13 times greater in the backwall flakes than in the host granite. -
-
•Salt crystallization pressures from growth of crystals from solution. Mixing salt cations and water can produce a supersaturated solution. When this solution evaporates, salt crystals precipitate in pores spaces. The resulting crystalline solid precipitated between mineral grains can exert stress and readily cause mineral breakdown.
Salt crystallization pressures are highly effective at physically, mechanically breaking grains apart. Bartrum (1936), Bradley, et al. (1978), Mustoe (1982) and many other tafoni researchers find that salt prizes apart mineral grains. A study by McGreevy (1985), who used a scanning electron microscope (SEM) to microphotograph salt crystals lodged between sandstone minerals, provided direct evidence that salt crystals physically dislodge grains.
How salt weathering works. Salt crystals preferentially press against confining walls of rock capillaries because they are not able to expand into smaller, unfilled, capillaries due to their atomic nature. The larger capillaries are preferentially filled because less chemical free energy is needed to fill larger spaces than smaller ones. After the larger capillaries become completely filled with salt crystals, the crystals are less likely to expand into unfilled smaller capillaries and instead exert force onto the surface of the confining walls. The pressure in the pore space caused by the precipitation of salt crystals increases as the crystals grow to the threshold of bond rupture (which varies by mineral type) or to the point that the chemical potential is raised high enough to promote salt crystal growth into smaller capillaries. For a detailed description of this process, see Trenhaile, (1987) or Wellman and Wilson, (1965). This process is analogous to water freezing in pore spaces and wedging grains apart.
Salt crystallization may not be a prerequisite for tafoni formation (Winkler, 1979; Norwick, 2003) because data of weathering of tafoni due to the pressures exerted by salt crystals is elusive. Pye and Mottershead (1995) suggested that the absence of actual salt crystals implies that salt is not essential in tafoni weathering processes or that weathering is chemical and not mechanical. Young (1987) and Mottershead and Pye (1994) show that salt, as a chemical weathering agent, may promote tafoni development by dissolving silica grains with an alkaline solution. In Australian sandstone, Young (1987) used scanning electron microscopy to reveal that sodium chloride chemically etches silica grains and that this alkaline solution increases the rate quartz dissolves.
Is salt essential? The typical occurrence of tafoni in high saline environments (e.g., arid deserts, intertidal areas) is strong evidence that salt likely plays a crucial role in tafoni formation. However, the explanation of salt weathering as a physico-chemical cavernous weathering agent is incomplete because it does not account for why tafoni develop in some but not all coastal rocks. Accordingly, in addition to salt, researchers emphasize additional controls and weathering agents that work to create tafoni.



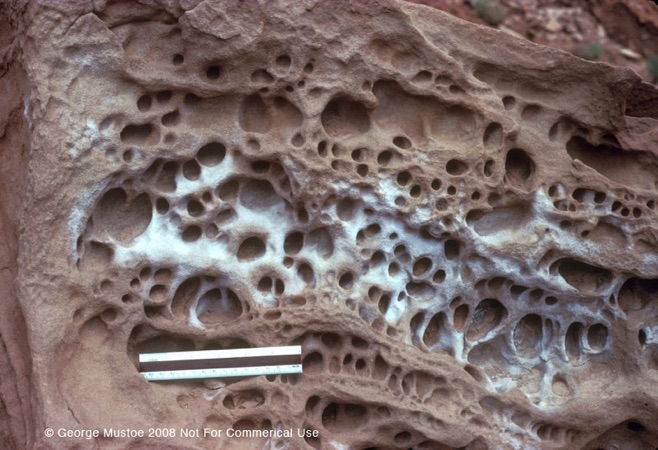
Sources. Salt can be delivered from external sources like sea spray and splash (Mustoe, 1982) and fog (Mottershead, 1994), with airborne salt cations providing the nuclei around which fog condenses, or salt can originate internally from the rocks themselves. Mottershead found that marine salts are most likely responsible for tafoni formation because, at his study site, chlorine concentration of the rock (greenschist) and the degree to which tafoni occupy rock surfaces are positively correlated. A well-recognized association connects the presence of tafoni and the occurrence of soluble salts such as sodium chloride, sodium sulphates, gypsum, epsomite, hexahydrite, sylvite, and calcium sulphates (Bradley, Hutton, & Twidale, 1978; Huinink, Pel, & Kopinga, 2005; Martini, 1978; Mustoe, 1982; Rodriquez-Navarro & Doehne, 1999; Selby, 1979; Young, 1987). Honeycomb in coastal environments may originate from a dynamic balance between the effects of salt weathering and the protective effects of biofilms (Mustoe, personal communication).